Shanghai Zhimeng Biopharma, Inc., founded in 2018, is a clinical-stage biopharmaceutical company dedicated to the discovery and development of novel small-molecule therapeutics for liver diseases and neurological/psychiatric disorders. As an innovative drug R&D platform focused on liver diseases, Zhimeng’s pipeline covers multiple stages of hepatitis B virus (HBV) treatment, including nucleocapsid assembly modulators to suppress viral replication, immunomodulators, and RNA destabilizers to inhibit HBV surface antigen production. The company aims to achieve functional cure for chronic hepatitis B (CHB) through comprehensive suppression of viral replication and protein expression while inducing effective antiviral immune responses. Beyond CHB, Zhimeng is also deeply engaged in developing innovative drugs for refractory epilepsy, neuropathic pain (e.g., cancer pain), amyotrophic lateral sclerosis (ALS), depression, and bipolar disorder. Several candidates have already entered clinical stages.
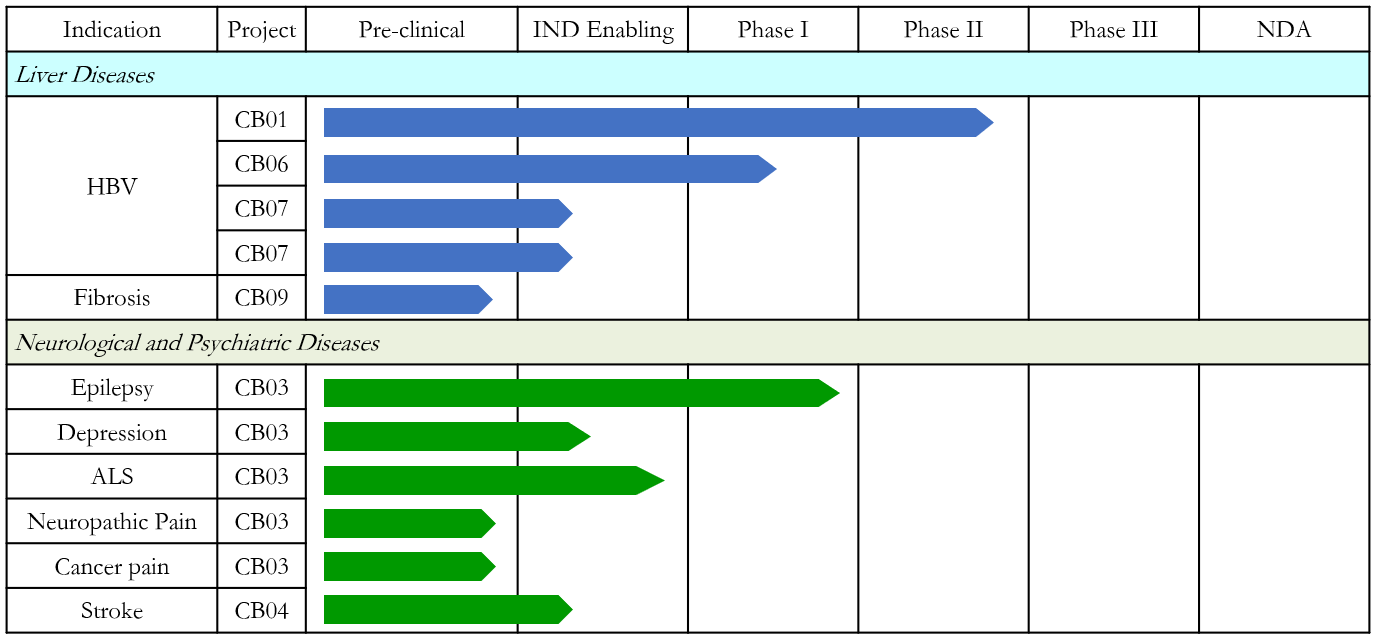
Chronic Hepatitis B Drug Development
Relevant data show that China has approximately 86 million HBV carriers, including about 30 million chronic hepatitis B (CHB) patients. However, the diagnosis rate is only 18.7%, and the treatment rate is less than 11%, far below the WHO 2030 targets (90% diagnosis rate, 80% treatment rate). In China, over 80% of hepatocellular carcinoma (HCC) cases are attributed to chronic HBV infection. While current nucleos(t)ide antiviral drugs can effectively control HBV replication and delay cirrhosis progression in most patients, a significant proportion exhibit suboptimal or no response. Moreover, these drugs rarely cure CHB, requiring long-term use and carrying the risk of viral rebound upon discontinuation. Thus, novel therapies targeting different stages of the HBV lifecycle are urgently needed.
Given the complexity of liver disease pathogenesis, Zhimeng’s strategy involves developing multiple novel and potent direct-acting antiviral agents to suppress HBV replication at various stages, combined with innovative immunomodulators to restore immune function, maximally inhibiting HBV cccDNA activity and clearing infected cells. Our goal is to deliver more effective, safe, and affordable innovative drugs for CHB functional cure, addressing unmet clinical needs and improving patients' quality of life.
HBV Capsid Inhibitor (ZM-H1505R)
The HBV core protein plays a critical role in the viral lifecycle. After infecting hepatocytes, core protein dimers assemble with pregenomic RNA/polymerase complexes to form nucleocapsids, where reverse transcription generates double-stranded DNA genomes. Drugs targeting core protein to disrupt HBV replication are a key focus in direct-acting antiviral development.
Nucleocapsid assembly modulators (CAMs) bind to hydrophobic pockets between core protein dimers, affecting capsid assembly and disassembly. CAMs not only interfere with normal capsid formation to suppress replication and reduce viral markers in peripheral blood but also inhibit capsid disassembly during de novo infection and intracellular replication, blocking reinfection and lowering total viral load in the liver.
ZM-H1505R is a novel pyrazole-class type II CAM, which is distinct from other investigational allosteric modulators. It induces the formation of replication-incompetent empty capsids, disrupting HBV replication. At higher concentrations, ZM-H1505R also inhibits de novo infection, reducing the formation of covalently closed circular DNA (cccDNA)the viral transcriptional template that persists as a minichromosome in hepatocyte nuclei. The inability of current HBV therapies to eliminate cccDNA is a major barrier to functional cure. Additionally, ZM-H1505R demonstrates excellent liver-targeting properties.
In Phase II trials, ZM-H1505R rapidly reduced HBV DNA to undetectable levels (plasma HBV DNA ≤10 IU/mL, complete virological response) in low-level viremia (LLV) CHB patients who had received long-term (e.g., >1 year) nucleos(t)ide analog therapy without achieving full response. The Phase II data also showed outstanding safety and tolerability. In January 2025, ZM-H1505R tablets were designated as a Breakthrough Therapy by China’s Center for Drug Evaluation (CDE) for the treatment of chronic hepatitis B.
TLR8 Agonist (CB06-036)
CB06-036 is an orally administered small-molecule TLR8 agonist independently developed by Shanghai Zhimeng Medicine Technology Co., Ltd. for the treatment of chronic hepatitis B virus (HBV) infection.
Toll-like receptor 8 (TLR8) recognizes pathogen-derived single-stranded RNA fragments to trigger innate and adaptive immune responses. Research indicates that chronic hepatitis B is associated with dysfunctional immune responses, making selective TLR8 agonists a potentially effective therapeutic strategy.
In preclinical studies, CB06-036 demonstrated excellent selectivity, potency, and safety. It induces cytokines in human peripheral blood mononuclear cells, which activate antiviral functions through multiple immune mediators. Additionally, CB06-036 exhibits favorable liver-targeting properties.
In April 2022, CB06-036 completed first-in-human dosing in a U.S. Phase I clinical trial. This randomized, double-blind, placebo-controlled study evaluated the safety, pharmacokinetics, and pharmacodynamics of single ascending doses in healthy adults. By June 2025, CB06-036 had completed a Phase Ib trial in chronic hepatitis B patients in China and New Zealand.
In November 2022, Zhimeng Medicine entered into a global exclusive licensing agreement with GSK for CB06-036. Following successful Phase I studies, GSK will develop, manufacture, and commercialize CB06-036. If successful, CB06-036 may enable functional cure through combination therapy or sequential treatment with bepirovirsen.
Neuroscience Drug Development
KCNQ2/3 Potassium Channel Opener (CB03-154)
CB03-154 is a potassium channel KCNQ2/3 opener independently developed by Zhimeng for treating refractory epilepsy, amyotrophic lateral sclerosis (ALS) in adults, depression, and bipolar disorder.
Epilepsy—affecting ~90 million people globally (9 million in China)—is characterized by seizures and transient neurological dysfunction. While antiseizure medications control most cases, ~30% of patients have drug-resistant epilepsy. Retigabine (Potiga®/Ezogabine®), the first FDA/EMA-approved KCNQ2/3 opener (2011), was withdrawn in 2017 due to vision impairment from retinal pigmentation. CB03-154, Zhimeng’s next-generation KCNQ2/3 opener, shows superior chemical/metabolic stability, selectivity, antiseizure activity, pharmacokinetics, and safety versus retigabine.
ALS is a fatal neurodegenerative disorder causing progressive skeletal muscle weakness and respiratory failure, with a median survival of 2–3 years post-symptom onset. Only four approved therapies modestly slow progression, leaving critical unmet needs. Motor neuron hyperexcitability is a key pathophysiological process in ALS. Riluzole, the first ALS drug, prolongs survival by reducing neuronal excitability but has limited efficacy. Research confirms that ALS hyperexcitability correlates with reduced Kv7.2/7.3 (KCNQ2/3) activity, and retigabine demonstrated potential to delay motor neuron degeneration.
CB03-154, Zhimeng’s proprietary next-generation Kv7.2/7.3 (KCNQ2/3) opener, significantly reduces motor neuron hyperexcitability in preclinical ALS models. It slows functional decline in muscle strength-related parameters, delays symptom onset, extends survival, and normalizes muscle/neuron morphology. Beyond symptomatic relief, CB03-154 shows potential to modify disease progression. In 2023, it received FDA Orphan Drug Designation for ALS. Zhimeng plans to initiate clinical trials for ALS in China.